By Anders Lorenzen
The rapid electrification of our society is a key component, for us to reduce emissions and create a more efficient economy.
But there are disadvantages in that. The scale-up of battery use could be more green, and there are also serious questions raised about the resources available for the key component, lithium-ion. The metal is most predominantly mined in South and Central America.
Lithium-ion batteries are the only batteries that, as yet, are produced at scale. They are found in everything from clean energy and tech applications to smartphones, computers, energy storage and so on – literally anything that requires a battery large or small.
But, the key component of those batteries, lithium, is considered a chemical element, included in the periodic table under the group of alkali metals, and is being used in so many different applications that it is deemed critical. Furthermore, it is estimated that only 5% of lithium-ion batteries are currently recycled. Therefore, scientists have been working at better ways to recycle them.
Some of the challenges of the current typical recycling methods require harsh liquid chemicals or heat to complete the process. This can produce toxic byproducts and requires large amounts of energy.
However, a team of US government scientists has developed a new system which could revolutionise the recycling process. This new method would eliminate the need for chemicals and high heat. The process would use the Battery Recycling and Water Splitting (BRAWS) technology which only uses water and CO2. And it also allows scientists to recover more lithium from used batteries than any other method.
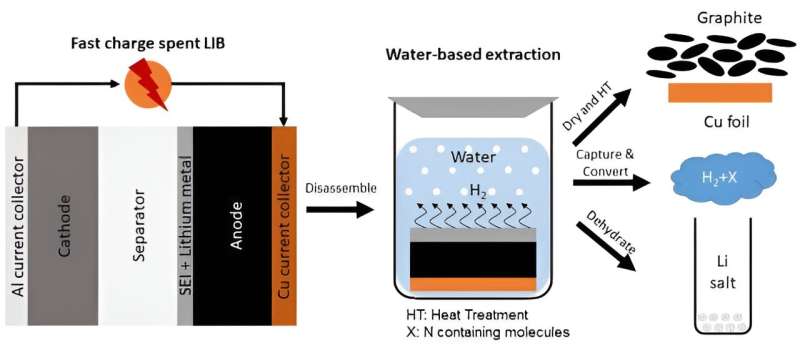
The process
When lithium-ion batteries undergo fast-charging, they do not last as long, because the fast- charging causes the lithium to build up on the anode (positive side of the battery electrode). Over time, the lithium build-up causes the battery to fail. The first step in the BRAWS technology is to use a set of protocols that includes fast-charging . This is to force as much additional lithium as possible to build up on the battery anode, then the battery is dismantled.
After dismantling the battery, the anode, typically made of graphite, is then immersed in water, and CO2 is added to recover lithium as lithium carbonate. This step results in the recovery of almost all the lithium from the original battery, and produces green hydrogen as a byproduct.
Ikenna Nlebedim, one of the scientists working on the project, explains that: “As lithium is very reactive when we put that anode in water, it divides the water molecule by stripping the oxygen and producing hydrogen as a gas, which can be recovered safely and used as a fuel.”
According to Nlebedim, the economic incentive in scaling this recycling method is this; that by recovering the lithium this way, other parts of the battery as well as enabling hydrogen production, you at the same strengthens the financial viability of the process.
Discover more from A greener life, a greener world
Subscribe to get the latest posts sent to your email.
Categories: clean tech, innovation, materials, Tech for Climate, technology